Unmasking Breast Cancer
 A new study from the Li Lab removes the cloak masking breast tumors, unleashing the body’s immune system to kill cancer cells
A new study from the Li Lab removes the cloak masking breast tumors, unleashing the body’s immune system to kill cancer cells
For the past 10 years, Arc Core Investigator and Stanford University Professor of Biochemistry Dr. Lingyin Li has been wrestling with a complex question: how can we make breast cancer more responsive to immunotherapy, a new and promising type of cancer treatment that enhances the body’s immune response to kill cancer cells? Today, only a small percentage (10-20%) of breast cancer patients respond well to immunotherapy, leaving most patients with few or no effective treatment options.
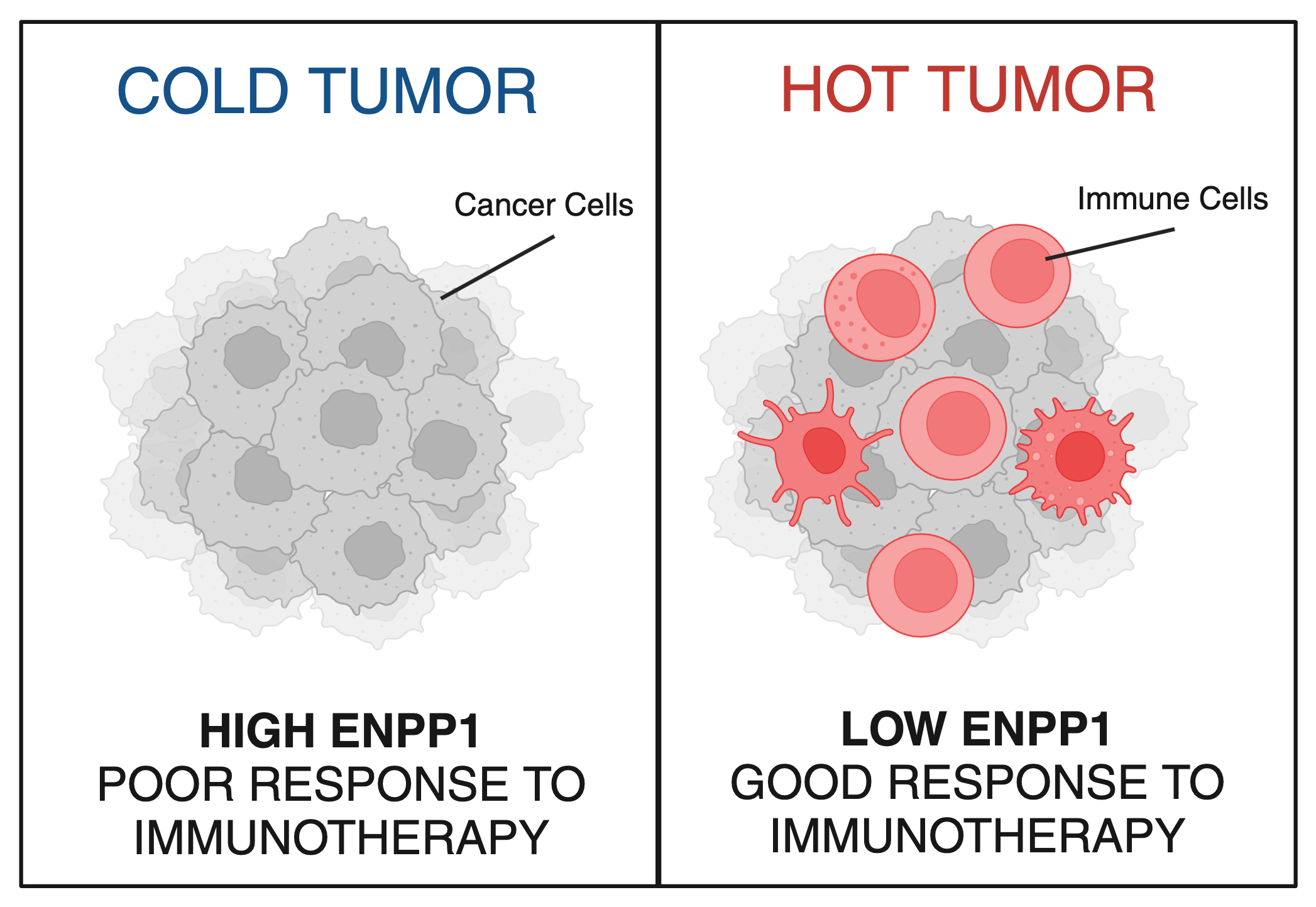 Now, new research from Li and colleagues, including Stanford MD-PhD student Songnan Wang, published in Proceedings of the National Academy of Sciences, reveals a critical determinant of breast cancer’s resistance to immunotherapy. Their findings show that a protein called ENPP1 acts like a mask, shielding cancerous cells from immune detection and enabling cancer to spread. Patients with high levels of ENPP1 have low immune response, do not respond to immunotherapies and have high chances of metastasis, while those with low levels of ENPP1 have high immune response, respond well to immunotherapies and do not go on to develop metastasis.
Now, new research from Li and colleagues, including Stanford MD-PhD student Songnan Wang, published in Proceedings of the National Academy of Sciences, reveals a critical determinant of breast cancer’s resistance to immunotherapy. Their findings show that a protein called ENPP1 acts like a mask, shielding cancerous cells from immune detection and enabling cancer to spread. Patients with high levels of ENPP1 have low immune response, do not respond to immunotherapies and have high chances of metastasis, while those with low levels of ENPP1 have high immune response, respond well to immunotherapies and do not go on to develop metastasis.
“There’s a huge unmet need in reducing the mortality rate of breast cancer,” said Li. “By identifying ENPP1’s role in suppressing the body’s immune response, our research is a significant step toward providing another effective treatment for patients living with breast cancer.”
Building a foundation of knowledge
Immunotherapies are revolutionary, Nobel Prize-winning treatments that encourage immune cells, particularly T cells, to keep fighting after tumors tell them to give up. But most breast tumors – in addition to other difficult-to-treat tumor types like pancreatic, ovarian, and prostate cancer – are what are known as “cold” tumors: these tumors are effectively invisible to the immune system, rendering them poor targets for immunotherapies like Keytruda.
A chemist by training and a biochemist by practice, part of Li’s work has focused on how to make “cold” tumors more responsive to treatment by turning them “hot.” In doing so, Li has made several pioneering discoveries that set her on the path to today’s new findings. The first demonstrated the promise of activating a molecular beacon - known as STING - to sound the alarm to recruit immune troops into the battleground of a tumor (1)
The second built on the discovery of the natural activator of STING in our bodies: a molecule called cGAMP that cells produce as a signal that something is wrong, such as a viral infection or cancer. Li discovered that cancer uses an enzyme called ENPP1 to chew up the cGAMP signal before it can sound the immune alarm through the STING pathway(2, 3).
Cancer biologists have long been linking high ENPP1 levels in many types of cancers to drug resistance and poor prognosis in patients. But it wasn’t clear which of ENPP1’s many activities in the body was responsible for its tumor-promoting ability, and if its degradation of cGAMP could be behind it.
Discovery across disciplines
“I vividly remember meeting Lingyin during my first visit to Arc and discussing where our two research worlds intersect. Through my own work to understand cancer metastasis, I had access to patient data that could ultimately shed light on the role ENPP1 plays in cancer metastasis - an avenue I had not explored myself,” said Dr. Hani Goodarzi, incoming Arc Core Investigator and Associate Professor at UCSF.
Interpreting clinical data analyzed by Goodarzi, Li and Wang determined that ENPP1 levels were highest in Stage 4 cancer patients – those whose cancer has spread to form metastatic tumors in other organs. While most labs had previously focused on primary tumors, this was the first clue that ENPP1 might be playing an outsized role in tumor metastasis.
Wang was inspired to recreate and extend this finding in mice. When she boosted the production of ENPP1 in breast cancer cells, the cancer started to metastasize faster - and when she turned ENPP1 down in highly aggressive cancer cells, she completely abolished metastasis. Most importantly, she showed that ENPP1 promotes breast cancer metastasis by degrading cGAMP to dampen the STING molecular beacon.
But Wang still needed to identify how ENPP1’s cGAMP-chewing ability works in complex tumor environments, where healthy cells surround a cancerous tumor. To unravel this, she collaborated closely with single-cell RNA sequencing experts in Dr. Luke Gilbert’s Lab and Arc’s Multi-Omics Technology Center, led by Dr. Nianzhen Li and Dr. Brian Yu, to get a high-resolution view of what each individual cell was doing in the mouse tumors. Wang showed that ENPP1 destroyed all the cGAMP produced by cancer cells before it could trigger the STING alarm in nearby healthy cells. In contrast, the tumors lacking ENPP1 effectively activated STING in surrounding cells and called in the immune cavalry, resulting in large numbers of activated immune cells arriving to fight the tumor.
This was a massive finding: it confirmed that ENPP1 enabled metastasis by suppressing cGAMP’s molecular signal in both cancer cells and the surrounding healthy cells, allowing cancer to hide from the immune system. Yet one major question remained: was ENPP1 masking human tumors from the immune system, just as it was in mice?
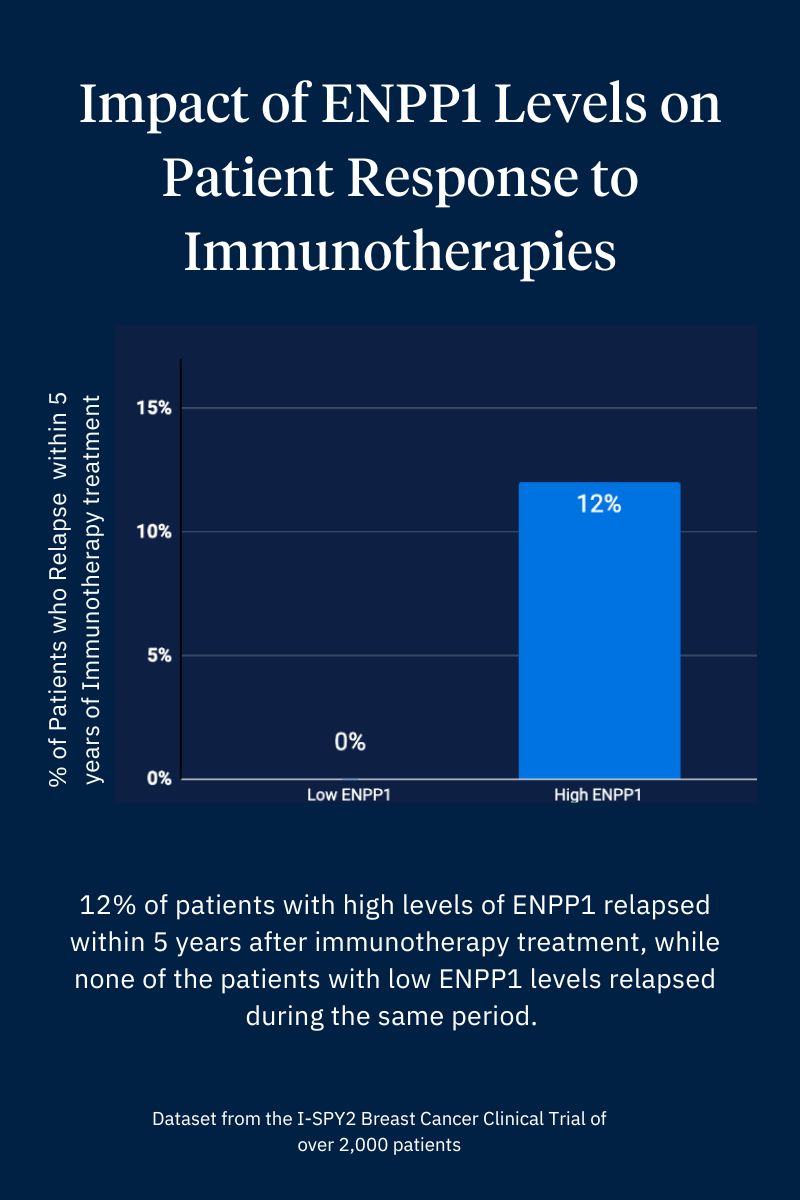 Li and Goodarzi decided to test Wang’s finding against a human dataset. With support from the ongoing I-SPY2 breast cancer clinical trial team, led by Dr Laura Van’t Veer at UCSF, Goodarzi dug into the I-SPY2 dataset from over 2,000 breast cancer patients.
Li and Goodarzi decided to test Wang’s finding against a human dataset. With support from the ongoing I-SPY2 breast cancer clinical trial team, led by Dr Laura Van’t Veer at UCSF, Goodarzi dug into the I-SPY2 dataset from over 2,000 breast cancer patients.
“The results were black and white: all of the patients with low ENPP1 responded to Keytruda and had no metastasis, while 12% of patients with high ENPP1 developed metastasis within 5 years,” said Li. “Without Songnan’s work of assembling the puzzle pieces together and seeking out collaboration with other researchers in our scientific community, we wouldn’t have gotten to this critical discovery.”
“Our findings showed that the ENPP1 gate keeps immune cells away from breast tumors in both mice and humans; it allows the cancer cells to grow and spread in our bodies while evading the immune system,” Wang explained. “By taking away the gatekeeper, we invite the immune system to intercept cancer cells and work alongside existing immunotherapies to eliminate them.”
Opening doors for immunotherapy
The Li Lab’s discovery now sets a foundation in place for the oncology community to begin measuring ENPP1 levels in order to select patients most likely to respond well to immunotherapy. In addition, looking forward, ENPP1 inhibitors, several of which are under early clinical development by Li’s group and other researchers, could make all patients responsive to immunotherapy, evening the playing field.
“Our research shows that curiosity-driven and collaborative scientific exploration can generate discoveries that inform clinical applications to make a real positive impact for patients,” Lingyin stated. “I hope our findings and approach inspire clinicians who treat cancer to further explore the implications of our research not just on breast cancer, but on other types of cancer.”
You can read the new paper in Proceedings of the National Academy of Sciences here, Stanford’s news story here, and learn more about the Li lab’s work here.
(1) Kim, S. et al., Anti-cancer flavonoids are mouse selective STING agonists. ACS Chem Biol **8**, 1396-1401 (2014).
(2) Li, L. et al., Hydrolysis of 2’3’-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol 10, 1043–1048 (2014).
(3) Carozza, J. A. et al., Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nat Cancer 1, 184–196 (2020).